Eight years ago, a team of students in the Master of Biomedical Innovation and Development program tried to address a pressing need in sports medicine: how to treat millions of patients with chronic soft tissue injuries that would not respond to physical therapy.
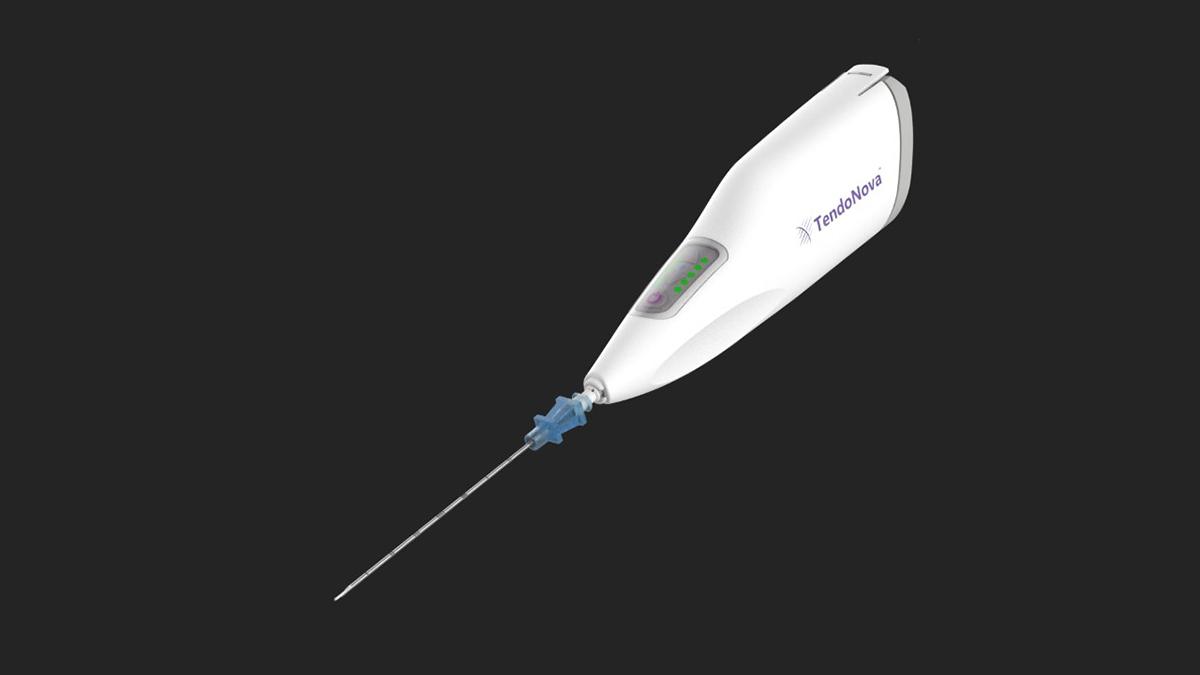
Their solution was a microinvasive surgical tool called the Ocelot, developed during the one-year master’s program known as MBID in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. By the end of this year, the Ocelot could be in the hands of physicians treating patients with chronic tendon pain.
TendoNova, the company the four former BME students started in 2017, recently announced that its Ocelot tool has received U.S. Food and Drug Administration 510(k) clearance, which means the company can soon begin marketing and selling its device. It will be manufactured in Georgia, a nod the company founders’ desire to keep the operation close to where their idea was born.
“The MBID program played a pivotal role in our journey by providing us with the fundamental tools and frameworks to translate an unmet need into a viable commercial idea,” said Luka Grujic, who co-founded TendoNova with fellow MBID grads Shawna Khouri, Brett Rogers, and Jonathan Shaw.
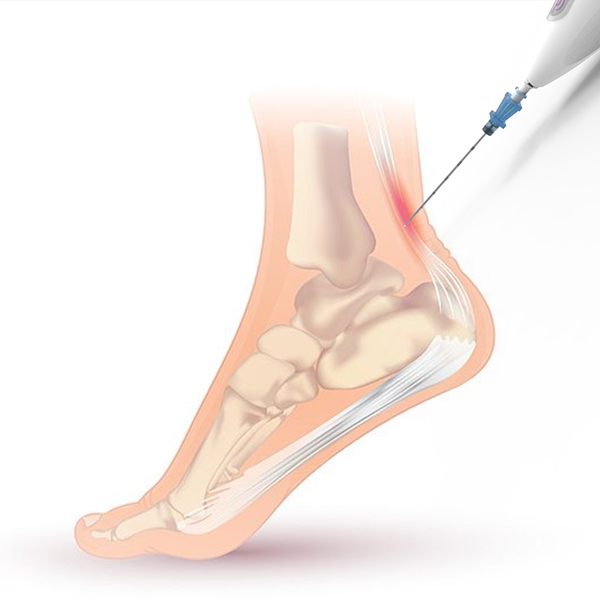
The Ocelot device is a handheld tool to perform mechanical fragmentation and debridement of targeted tissues, a common procedure for athletes and others suffering from tendon pain disorders like tennis elbow, plantar fasciitis, and jumper’s knee. According to the Centers for Disease Control and Prevention, more than 30 million people in the U.S. suffer from chronic tendon pain, and half of them have little or no relief. The fragmentation and debridement procedure encourages growth of healthy tendon to replace the painful tissue.
The need for a new approach to the procedure initially came to the team from Ken Mautner of Emory Sports Medicine, who is head physician for the Atlanta Braves and Atlanta Hawks. Shaw, a physical therapist at the time, brought Mautner’s needs to his teammates, who started thinking about in-office, ultrasound-guided tools.
“The initial Ocelots built during the MBID program were rough, quick-turn prototypes focused on demonstrating basic functionality and understanding the needs of the clinician,” Grujic said.
The first prototype was basically a beefed-up tattoo gun. Except, instead of an oscillating needle penetrating the top layer of skin with ink, this model went beneath the skin to reach the pathological tissue.
“While in the MBID program, we worked to combine clinical functionality with refinement on the human factors,” Grujic said. “We used inspiration from the tattoo gun’s human factor design to make the Ocelot usable with a single hand and a pencil grip, since both the tattoo gun and our device require precision and accuracy when being used.”
The team made modifications, such as adding a cannula around the oscillating component to protect surrounding tissue, making the device more tube-shaped for easier maneuverability inside the body, and lowering the noise with different hardware to reduce patient anxiety.
At the end of the MBID program, the team had a prototype that represented a vision of what the Ocelot device and TendoNova could become. MBID Program Director Sathya Gourisankar wasn’t surprised by the team’s rapid progress.
“They displayed enormous aptitude for innovation from the outset and made tremendous progress on identifying the relevant unmet clinical need, followed by concept phase development, and finalizing it to a viable bench to bedside prototype — all within three semesters of the MBID program,” Gourisankar said.
As students, they benefited from the mentorship of MBID faculty with extensive medical technology commercialization experience and business courses that equipped them with project management and business strategy know-how. The program’s resources made the team a compelling choice for grants from the Georgia Research Alliance and the NFL Players Association to continue developing their idea.
“We were able to integrate and appropriately strategize design decisions to navigate the barriers of regulatory, reimbursement, and device standards from the ground up,” Grujic said. “Some of the most unique and valuable aspects of the MBID program are the industry instructors and the ability to take classes at the Scheller College of Business, a unique blend of expertise for a master's program.”
In the years since finishing their master’s degrees, the team has taken its handheld surgical device from its final prototype into a full-blown system. Based on testing and feedback from TendoNova’s growing list of medical advisors, the device has been completely redesigned “from the inside out, from improving the internal mechanisms, battery life, and motor performance, to designing for manufacturability,” Grujic said.
The group also added features such as wireless charging, variable speed selection, a detachable Luer lock, and the ability for the clinician to see how the Ocelot is performing in real time through an app that is included with the system.
“This FDA clearance is a testament to following through on all the work required to bring the idea from the MBID program to the marketplace,” said Grujic, now a senior global product manager for Medtronic. His teammates also have other jobs beyond their roles as medical device entrepreneurs. Khouri is virtual health manager for Tulsa Innovation Labs, Shaw is director of sales for OXOS Medical, and Rogers is director of operations and engineering for Facet Medical Technologies.
Together, they put in a lot of nights and weekends while working their full-time jobs to build TendoNova and get to this point, according to Grujic, and last year brought on as CEO Mark Samuels, an experienced technology company executive and entrepreneur.
“The work ethic of this team has been truly amazing,” Gourisankar said. “But what I was most impressed with was the team’s conviction from the early stages that this was a real product that was going to have an impact in clinical settings on a patient’s well-being, as opposed to just an academic project. I’m sure they’re going to build a platform of technologies around their concepts and shape the future of surgical care in these areas in the years to come.”
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.