Which door is the right door? New research from Annabelle Singer’s lab has described for the first time the role of what are called “non-place cells” in distinguishing between the goal we’re trying to reach when navigating and a similar-but-not-quite-right goal. Their findings are published in the journal Proceedings of the National Academy of Sciences. (Image: Arek Socha via Pixabay)
Visitors to Atlanta often are confounded by the various and sundry streets, roads, places, and drives named Peachtree (never mind the northeast, southwest, or other directional variations). Writers and late-night comedians are sure to poke fun at the region’s affinity for the name any time there’s a big event in town — and, to be sure, it can make navigating Atlanta tricky.
A new study from researchers at Georgia Tech and Emory University is shedding new light on how our brains make sure we’ve reached the right road named Peachtree. And it turns out, the neurons that help us distinguish between our navigation goal and a close-but-not-quite-right goal also are impaired in models of Alzheimer’s disease.
Published in the Proceedings of the National Academy of Sciences, the paper from Annabelle Singer’s lab in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University explains how a group of cells in the brain called non-place cells play a vital role separating the real destination, or goal, from a false one. They work in tandem with neurons in the brain’s hippocampus called place cells that help us understand where we are in space — and these non-place cells don’t look like what neuroscientists might have expected.
“Before our paper, the view is that, during navigation, there are cells that code for place, and then there are cells that do this conjunctive coding — essentially, goal plus place — but there aren’t cells dedicated purely to coding for reaching the goal,” Singer said. “And don't you think you would want that?
“These cells [we studied] are not goal cells in the way that we might have imagined them — that they fire when you reach the goal. Rather, they fire differently between the goal and the false goal.”
The study described for the first time these non-place neurons’ unique role in separating a real goal from something that looks like the goal. Though previous studies suggested these cells played a role in spatial orientation alongside place cells, Singer’s team showed they do something additional that place cells don’t do.
“When we do the population-level decoding of neurons and these are included, we get an accurate representation of where you are in terms of discriminating between the real and the false goal, or the real goal and the rest of the track,” said Singer, McCamish Foundation Early Career Assistant Professor in the Coulter Department. “But when you exclude the non-place cells, now we can't do that decoding anymore — we can't separate the goal and the false goal.”
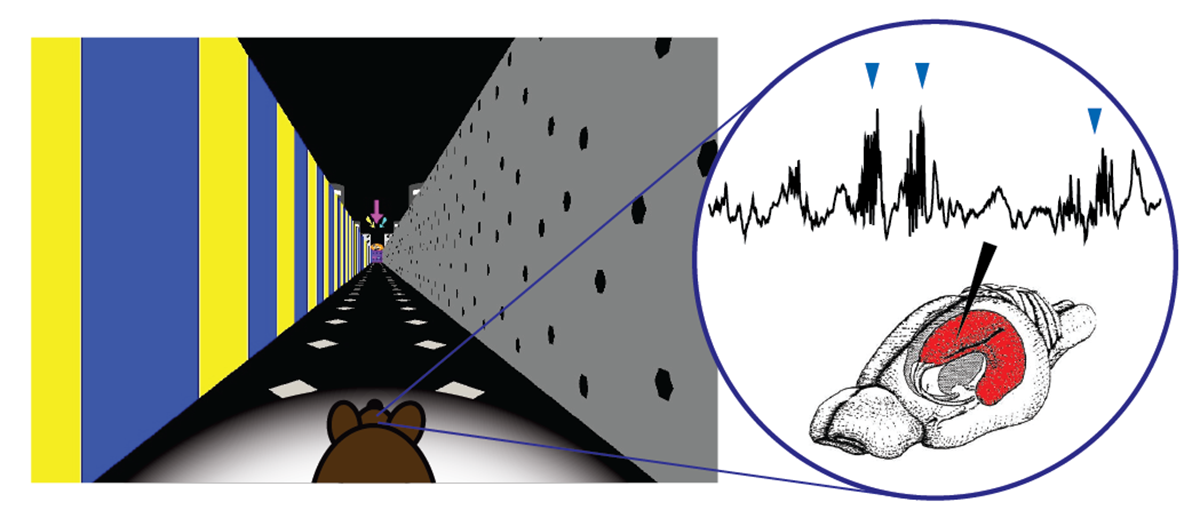
The team — including postdoctoral fellow Lu Zhang and Ph.D. students Stephanie Prince and Abigail Paulson — was comparing healthy mice and mice with Alzheimer’s disease pathology as they navigated a tiny virtual-reality maze in search of a treat. The mice run on a floating ball while a digital track is projected on screens around them. Some of the paths looked similar to the treat’s ultimate location, but with no treat at the destination.
As they looked at the oscillations of electrical signals in the brain, the researchers found non-place cells were out of sync in the Alzheimer’s models and also in healthy mice when they failed to reach the correct goal — and receive the treat.
Singer credited Zhang for persistently digging to understand their serendipitous discovery.
“We just kept trying to figure out what these cells were doing, and that's how we realized, they're not place cells, but they're doing something related to discriminating between the real goal and this cue that that looks like the goal,” Singer said. “It is a testament to how hard Lu, the first author, worked to push this all the way, to keep going and asking: What does that mean? What are the players? They're not coding what we think, so what are they doing?”
People with Alzheimer’s disease often have early deficits in spatial navigation, so the impairment of these non-place cells in the mouse models of the disease could partly explain those issues. Making that connection, though, requires further work.
“If you just took the traditional approach to studying these cells, you would ignore them, because they don't have a clear firing response,” Singer said. “So, we ended up coming at them kind of backwards.”
This research was supported by the Packard Foundation, the National Institutes of Health, grant Nos. R01 NS109226 and NRSA 5F31AG066410; and the National Science Foundation, grant No. DGE-1444932. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any funding agency.
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.