To learn how to make an artificial heart, Harvard and Emory/Georgia Tech researchers have made artificial fish.
The fish are made from human cardiac muscle cells, painstakingly grown on laboratory tape coated with gelatin. The “biohybrid” fish swim by recreating the muscle contractions of a pumping heart, bringing engineers closer to creating more complex artificial pumps and providing a platform to study human diseases, such as cardiac arrhythmias.
Biomedical engineer Sung Jin Park began working on biohybrid fish as a postdoc in Kit Parker’s lab at Harvard’s Paulson School of Engineering and Applied Sciences. Park is co-first author of a paper in Science, published online Feb. 10. Keel Yong Lee, a postdoctoral fellow at Harvard, is also co-first author.
“Our major achievement in this paper is that we can build a living muscle that can self-control and self-pace its movement without any additional control mechanism,” said Park, now an assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory and part of the Multi-Cellular Engineered Living Systems (M-CELS) program.
This artificial “biohybrid” fish is made with commercially available human heart cells and swims by recreating the muscle contractions of a pumping heart. Published in Science, the system brings engineers closer to complex artificial pumps that could grow with young patients. It also provides a new platform for studying heart rhythm problems. (Photo: Michael Rosnach, Keel Yong Lee, Sung-Jin Park, Kevin Kit Parker)
Park said his team’s research could be applied to design “biological pacemakers,” a potential alternative to electronic cardiac pacemakers. As part of an long-term implant, biological pacemakers could grow along with a pediatric patient.
“Our ultimate goal is to build an artificial heart to replace a malformed heart in a child,” said Parker, senior author of the Science paper. “Rather than using heart imaging as a blueprint, we are identifying the key biophysical principles that make the heart work, using them as design criteria, and replicating them in a system — a living, swimming fish — where it is much easier to see if we are successful.”
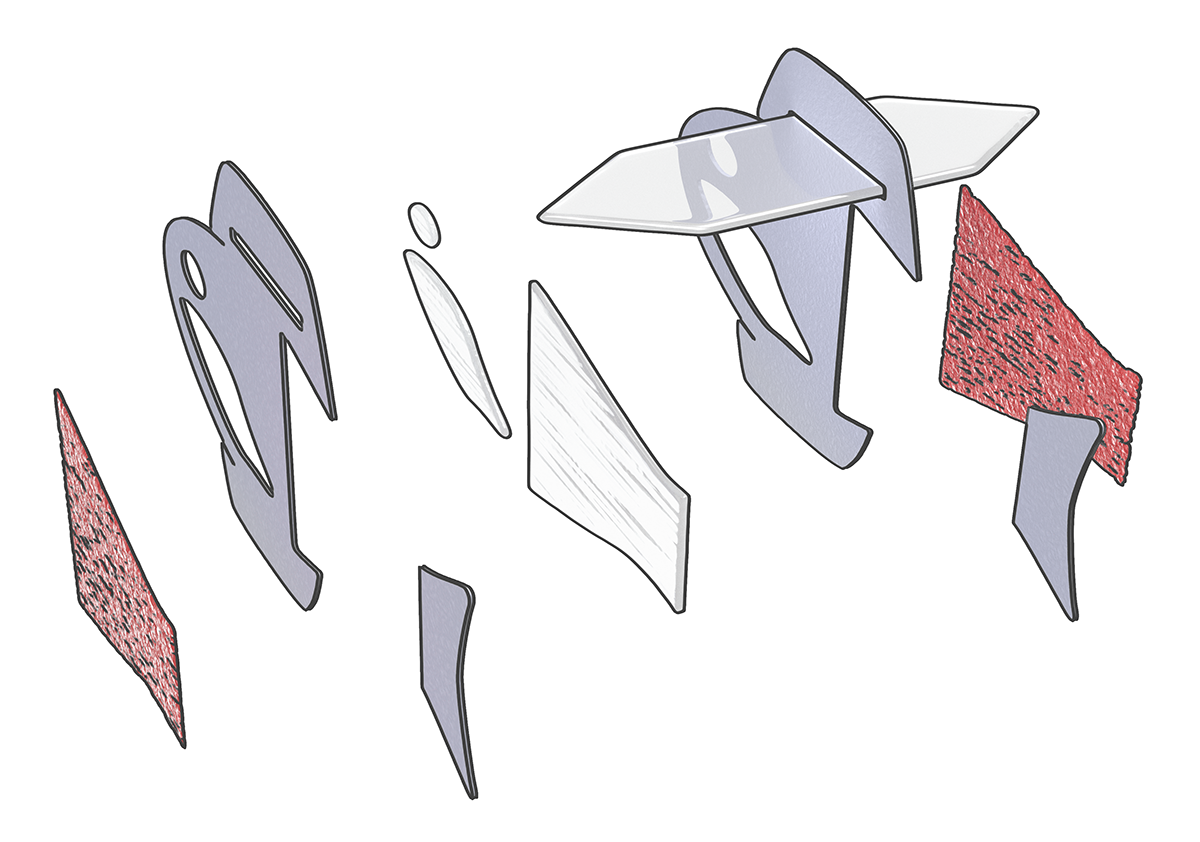
The fish has two layers of muscle cells, one on each side of the tail fin. When one side contracts, the other stretches. The stretch triggers the opening of an ion channel sensitive to mechanical movement, leading to an influx of charged ions and a contraction on that side.
The researchers also engineered an “autonomous pacing node,” acting like a pacemaker, which controls the frequency and rhythm of the spontaneous contractions. Together, the two layers of muscle and the autonomous pacing node enable the generation of continuous, spontaneous, and coordinated, back-and-forth fin movements. The system can propel the fish for more than 100 days.
In addition, the biohybrid fish improves with age. Its muscle contraction strength, maximum swimming speed, and muscle coordination all increase for the first month as the cardiomyocyte cells mature. Eventually, the biohybrid fish reached speeds similar to a model organism (zebrafish) in the wild.
The biohybrid fish builds off previous research from Parker’s lab. In 2012, the Harvard team used cardiac muscle cells from rats to build a jellyfish-like biohybrid pump, and in 2016 Park and his colleagues developed a swimming, artificial stingray, also from rat heart muscle cells.
The team’s discovery of the self-generated stretch/contract cycles was partly fortuitous.
“We did not expect that the mechano-electric coupling effect would be strong enough to produce our fish’s antagonistic muscle contractions,” Park said. “In the beginning, we used light to control fish movements, like what we did for our stingray. One day, after finishing this experiment, we saved the fish in the incubator for more experiments but totally forgot the fact that we saved the fish. After a couple of weeks, when we opened the incubator, we found out that the fish swam with its own pacing by itself.”
Park said he is eager to work on models of cardiac arrhythmias (sinoatrial node dysfunctions) and wants to investigate mechano-electrical signaling as a therapeutic target of heart rhythm management.
The human cardiac muscle cells used in this research are commercially available. They come from induced pluripotent stem cells, which are originally derived from skin cells.
The research was supported by the National Center for Advancing Translational Sciences (UH3TR000522, UG3HL141798) and the National Science Foundation (DMR-1420570). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any funding agency.
An illustration showing the five-layered body architecture of the biohybrid fish. The fish has two layers of muscle cells, one on each side of the tail fin. When one side contracts, the other stretches, creating a self-sustaining swimming motion. (Image: Michael Rosnach, Keel Yong Lee, Sung-Jin Park, Kevin Kit Parker)
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.