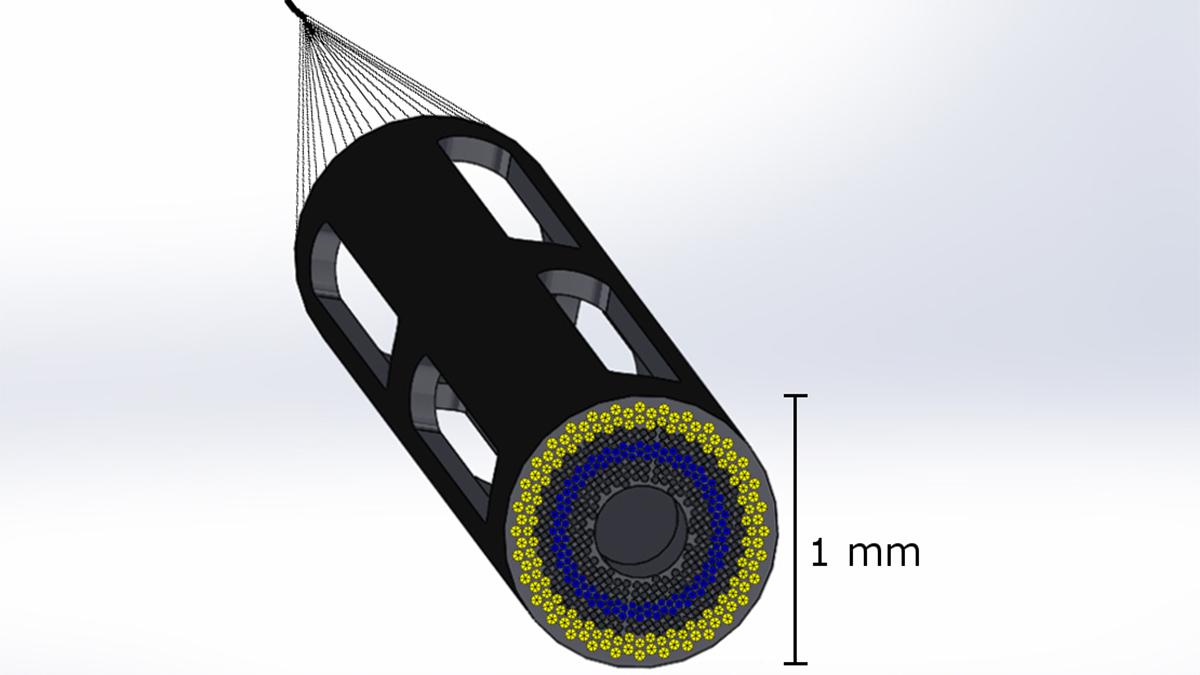
Proposed design of a 1mm catheter-based ultrasound device designed to simultaneously measure plaque composition, artery structure, and hemodynamics in 3D in coronary arteries. The device minimizes disturbances to blood flow from the catheter itself. Assistant Professor Brooks Lindsey is leading a new $2.5 million project to develop the device, which will help doctors assess whether patients need aggressive treatment to prevent a heart attack. (Image Courtesy: Brooks Lindsey)
Cardiovascular disease is the leading cause of death in the United States, and coronary artery disease specifically is responsible for 366,000 deaths each year, according to the Centers for Disease Control and Prevention. Despite the widespread impact of coronary artery disease, gaps in information make treating the condition a challenge.
With the support of a four-year, $2.5 million grant from the National Institutes of Health, Brooks Lindsey will lead a team developing an imaging system to fill these gaps and guide treatment based on assessing the risk of heart attack in patients with coronary artery disease.
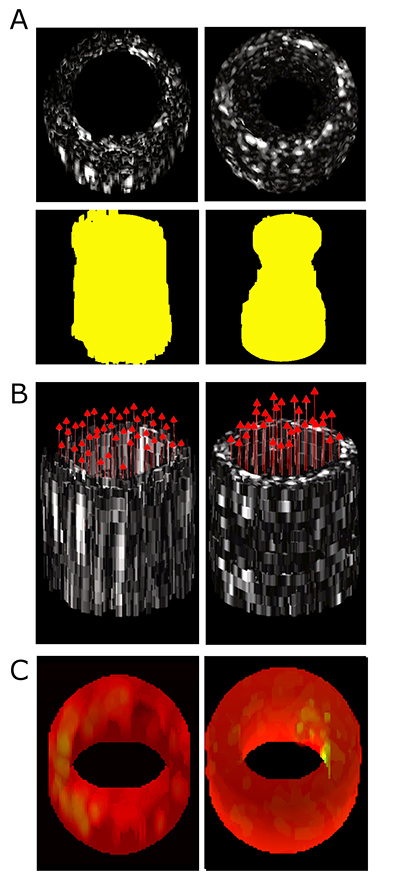
An example of the kind of simultaneous data collection that will be possible with the new catheter-based ultrasound device. This figure shows 3D functional imaging with a prototype forward-viewing device in laboratory models of narrowed vessels: (A) Conventional ultrasound imaging of lesion morphology in straight and stenotic, or narrowed, vessels, with extracted lumen shape shown in yellow, (B) 3D vector velocity imaging in straight and stenotic vessels, and (C) Strain rate imaging of a control vessel model and a model with a soft inclusion on the right inner surface. The proposed system to be built under this project will be smaller and have higher resolution than this prototype. (Image Courtesy: Brooks Lindsey)
Many of those patients are treated via a minimally-invasive procedure that places a stent to re-open arteries that have become narrowed with plaques. Partially occluded coronary arteries can result in heart attack in some of these patients. However, other patients have a similar blockage but have stable disease and do not require intervention. The challenge is deciding which patients are which.
“In the cardiac catheterization lab where these procedures are performed, there are a number of separate approaches for quantifying functional markers, such as local blood pressure, blood flow velocity, and plaque composition. However, all of these tools function independently and in isolation from one another,” said Lindsey, assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “Most are one-dimensional measurements, which makes it difficult to measure everything going on in the complex, 3D, local biomechanical environment. This includes tissue and plaque mechanical properties, artery geometry, and hemodynamics, all of which vary dynamically as the heart beats.”
While current tools can measure blood flow velocity or blood pressure and characterize plaque composition independently, all of these factors together contribute to the likelihood of plaque rupture and heart attack. No current method can acquire all this information with spatial and temporal information intact.
Lindsey’s lab will address this gap by developing a tiny ultrasound imaging device approximately 1 millimeter in diameter to measure these properties in 3D from the tip of the catheter during procedures in the cardiac catheterization lab. Their approach will be designed to allow simultaneous measurement of blood flow velocity, mechanical properties of tissue, and artery geometry for the first time.
“More than 1 million cardiac catheterizations are performed each year in the U.S.,” Lindsey said. “Even patients who ultimately do not require intervention undergo diagnostic catheterization, but there is no way to measure all the properties simultaneously. The goal of this project is to develop a system that uses ultrasound on the tip of catheter to give cardiologists a complete picture of the patient’s individual anatomy and physiology, including dynamic behavior in coronary arteries as the heart beats. This imaging information, in turn, allows development of improved computational models of coronary arteries in health and disease.”
Lindsey will lead engineering efforts, including development of the imaging device and algorithms to quantify hemodynamics. Clinical aspects of the project will be handled by Habib Samady, a cardiologist at Northeast Georgia Medical Center in Gainesville who is an expert in imaging hemodynamics in clinical practice. Alessandro Veneziani, professor in Emory’s Department of Mathematics and Computer Science, will lead computational modeling efforts.
Muralidhar Padala, director of the Structural Heart Research & Innovation Program and associate professor in Emory’s Division of Cardiothoracic Surgery, will lead testing in coronary artery disease models. Coulter BME Professor John Oshinski will provide expertise in imaging-derived fluid dynamics.
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.