Low-density lipoproteins, or LDLs, usually get a bad rap. It’s because LDL particles (also known as “bad cholesterol”) are directly involved in the development of atherosclerosis, thickening of the artery wall which often leads to fatal cardiovascular disorders, like stroke or heart attack.
But LDLs also help serve a number of beneficial and necessary roles, including synthesizing Vitamin D in the skin from sunlight exposure, and aiding in the communication of neurons. The main function of LDLs involves the transportation of cholesterol from the liver to other tissues, to facilitate the formation of cell membranes.
These mobile little particles also taxi triglycerides, phospholipids, antioxidants, fat soluble vitamins, and proteins. And LDLs, which home-in on LDL receptors in cells, may be the perfect vehicle for another, potentially life-saving payload: In a number of studies, they’ve shown promise as nanocarriers for the precision deployment of cancer drugs.
“Since most normal cells do not express as many LDL receptors as tumor cells do, LDLs can be considered a good candidate for targeted drug delivery,” notes Chunlei Zhu, the lead author of a recently-published research paper in Angewandte Chemie (a peer-reviewed journal of the German Chemical Society).
Zhu was a postdoctoral researcher in the lab of Younan Xia, professor in the Wallace H. Coulter Department of Biomedical Engineering and researcher in the Petit Institute for Bioengineering and Bioscience who supervised the research and co-authored the paper, “Reconstitution of Low-Density Lipoproteins with Fatty Acids for the Targeted Delivery of Drugs into Cancer Cells.”
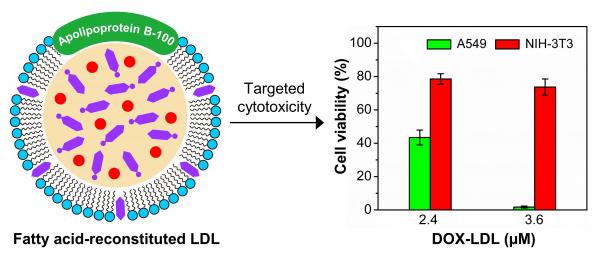
While this isn’t the first study to demonstrate the potential of reconstituted LDLs for targeted drug delivery, the contribution from Xia’s lab lies in the development of a new class of promising biomaterials in the form of naturally occurring fatty acids, which enable drug loading and release.
One way to introduce a therapeutic payload is encapsulation in the hydrophobic core of LDLs, which seems well-suited to drug delivery because of the simple procedure and relatively large loading capacity. Basically, it’s like redesigning and rebuilding the interior of a custom delivery van for better performance, at the molecular level.
The conventional encapsulation process involves use of a nonpolar organic solvent (heptane) to extract the hydrophobic lipids from the core, which is then refilled with a mixture of cholesterol esters and drugs, or cholesterol-drug conjugates.
“However, the presence of excess cholesterol in the bloodstream greatly increases the risk of forming atherosclerosis plaques in the aortic vasculature,” notes Zhu, who has returned to China to take a university faculty position.
In the study from Xia’s lab, researchers replaced the endogenous core lipids with a mixture of lauric and stearic acids, which serve as a matrix for drug loading, and provide several advantages: First, they’re naturally occurring biomolecules, so they serve as biocompatible and biodegradable hydrophobic environment for hydrophobic drugs. Fatty acids also are an important fuel source that may enable metabolism-triggered drug release. They can protect the drug payloads from hydrolysis. And there’s an added bonus: lauric acid has a favorable effect on the increase of “good cholesterol” (high-density lipoprotein, or HDL), and stearic acid enables the decrease of “bad cholesterol,” or LDL, in the bloodstream.
“As such,” the authors write, “these naturally occurring compounds can be considered safe materials for the substitution of cholesterol ester in LDL reconstitution.”
In addition to Zhu and Xia, contributing authors include Petit Institute researcher Krish Roy (professor in the Coulter Department and director of the Marcus Center for Cell-Therapy Characterization and Manufacturing), Pallab Pradhan (research scientist in Roy’s lab), Da Huo and Jiajia Xue (postdoctoral fellows in Xia’s lab), and Song Shen (associate professor at Jiangsu University in China and a visiting scholar in Xia’s lab).
Media Contact
Jerry Grillo
Communications Officer II
Parker H. Petit Institute for
Bioengineering and Bioscience
Keywords
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.