Contrary to common medical guidance, chemotherapy does not appear to be the only culprit in neuropathy, a neurological side effect of cancer treatment, a new study says. Cancer itself contributes heavily, too, and the stresses on neurons appear far worse than the sum of the two causes.
“There was some distress caused by cancer alone and some distress from chemo alone, but when you put the two things together, it was off the charts, seven times the trauma to neurons of the two things added together,” said Nick Housley, first author of the study performed in rats at the Georgia Institute of Technology. “It turned out to be the first-ever evidence that there is this exacerbation going on.”
Every year in the U.S., there are 1.8 million new cancer diagnoses, and about half of patients receive platinum-based drugs, which are very effective. About 40 percent of patients receiving platinum chemotherapy come down with neuropathy, suffering strange sensations, pain, fatigue, or loss of muscle coordination that impedes day-to-day life. Neuropathy can persist for years after chemotherapy ends.
Hope for treatment
The new study also challenges established medical explanations that neuropathy is caused by structural damage to nerves alone and that it is untreatable.
“The idea of damage has been the standard explanation – that these neurons are dying back and that they are retracting. And we have found time and time again zero evidence of this in the neurons we studied here,” Housley said.
“The evidence does not support physical damage as the basis for the disabilities we observe. We find functional problems that might be fixable,” said Tim Cope, one of two principal investigators of the study. Functional problems usually refer to neurons not firing properly versus actual tears or shrinkage in neurons.
Cope is a professor in Georgia Tech’s School of Biological Sciences and in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. Housley is a postdoctoral researcher in his lab.
The researchers published their results in the journal Cancer Research on April 28, 2020. The research was funded by the National Institutes of Health’s National Cancer Institute. John McDonald, the other principal investigator, is a professor in Georgia Tech’s School of Biological Sciences and the director of Georgia Tech’s Integrative Cancer Research Center.
Gene expression surprises
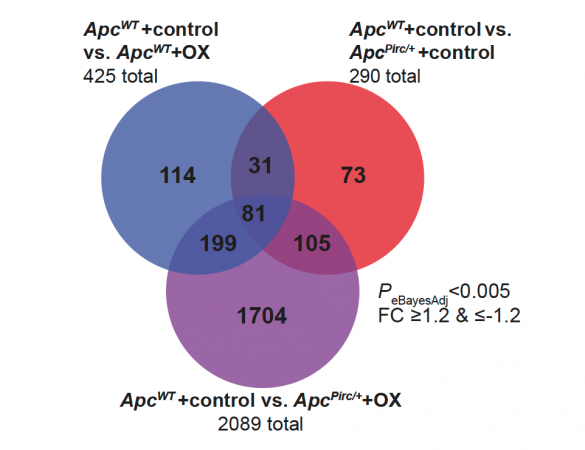
To explore neuropathy’s causes, the researchers cast a broad net. They examined gene expression in neurons, protein expression, and neuron signaling, and they measured body movements, which became markedly uncoordinated.
Gene dysregulation went into overdrive in reaction to chemo and cancer, including boosting inflammatory responses while suppressing some of neurons’ protective mechanisms. But despite the plethora of gene regulation red flags, there were also surprising signs of intact neuron health.
“Many things the neuron relies on to live and function were unscathed on the gene expression level. That’s potentially good news for patients and for fixing neuropathy because it means there may be just one thing or a few things to fix to restore normal functioning,” Cope said. “The downregulation of just a few genes may be responsible for the problems we’ve seen.”
Ion channel mystery
Clues from one downregulated gene took a twist that led to a new scientific discovery in neuronal biology before arriving at what appeared to be a pathology.
The downregulated gene is responsible for creating protein structures called ion channels that appear in a neuron’s cell wall and enable the neuron to fire electrical impulses, i.e. action potentials. Ion channels abruptly shuttle potassium ions (K+) and sodium ions (Na+) in and out of cells, flipping the net negative and net positive charges on either side of cell walls, which creates the action potentials.
This particular downregulated gene was for a potassium ion channel called Kv3.3, which was not previously known to exist in muscle spindles – neural receptors embedded in muscles to sense when they are contracting or stretching. The researchers found the channel to be prolific there.
“That was a discovery in its own right in basic neuroscience. Finding its involvement in this sensory-motor problem was also profound,” Housley said.
Most Kv3.3 expression disappeared under the combination of chemo and cancer. More research is needed to establish whether the lack of Kv3.3 is indeed an important contributor to neuropathy, but in this study, it strongly correlated with observed neural pathology.
“Despite the neurons still having the ability to fire action potentials, the process of neurons encoding information was really corrupted,” Housley said.
Also read: App Detects Harsh Side Effect of Breast Cancer Treatment
The following researchers from Georgia Tech coauthored the study: Paul Nardelli, Dario Carrasco, Travis Rotterman, Emily Pfahl, and Lilya Matyunina. The research was funded by the National Institutes of Health’s National Cancer Institute (grants R01CA221363 and R01HD090642). Any findings, conclusions, and recommendations are those of the authors and not necessarily of the NCI.
Here's how to subscribe to our free science and technology email newsletter
Writer & media inquiries: Ben Brumfield (404-272-2780), ben.brumfield@comm.gatech.edu or John Toon (404-894-6986), jtoon@gatech.edu.
Georgia Institute of Technology
Media Contact
Keywords
chemotherapy Chemotherapy Side Effects Neuropathy Neurological Side Effect Platinum Chemotherapy Fatigue Fatigue And Cancer Fatigue And Chemotherapy Fatigue And Breast Cancer Muscle Coordination Strange Sensations neuron firing Neuron Health National Cancer Institute Gene Dysregulation Inflammatory Responses ion channel Ion Channel Protein Potassium Channels Kv3.3 Muscle Spindle Spindle
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.